Difference between revisions of "Phosphorus"
Jenny Hill (talk | contribs) |
Dean Young (talk | contribs) |
||
| (54 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | === | + | [[File:Phosphorus Cycle copy.jpg|thumb|500px|Phosphorus Cycle from Wikimedia Commons]] |
| − | + | {{TOClimit|2}} | |
| − | ---- | + | |
| − | === | + | ==Phosphorus in stormwater== |
| − | + | Phosphorus in stormwater exists in both particulate and dissolved forms and is often explained in terms of total phosphorus (TP) and orthophosphate (–PO<sub>4</sub><sup>3-</sup>). Total phosphorus is the sum of particulate and dissolved phosphorus and contains both organic and inorganic forms. Particulate phosphorus is often associated with solids and sediments. Dissolved phosphorus consists of mainly inorganic orthophosphate and organic phosphorus. Orthophosphate meanwhile indicates the bioavailable phosphorus. | |
| − | {{:Phosphorus testing}} | + | |
| − | ---- | + | Phosphorus in stormwater is commonly considered to be primarily available in particulate form. While most studies reported majority of phosphorus (~55%) in particulate form (Maestre and Pitt 2005<ref name="example1">Maestre, A., and Pitt, R. (2005). “The National Stormwater Quality Database, Version 1.1 a compilation and analysis of NPDES stormwater monitoring information.” U.S. Environmental Protection Agency (EPA) Office of Water, Washington, DC.</ref>; Erickson et al, 2012<ref>Erickson, A. J., Gulliver, J. S., and Weiss, P. T. (2012). Capturing dissolved phosphorus with iron enhanced sand filtration. Water Res., 46(9), 3032–3042.</ref>) in some cases the dissolved form may explain up to 90% of the total phosphorus (Erickson et al. 2007<ref>Erickson, A., Gulliver, J. S., and Weiss, P. T. (2007). “Enhanced sand filtration for storm water phosphorus removal.” J. Environ. Eng., 10.1061/(ASCE)0733-9372(2007)133:5(485), 485–497.</ref>; Kayhanian et al. 2012<ref>Kayhanian, M., Fruchtman, B., Gulliver, J. S., Montanaro, C., Raniere, E., and Wuertz, S. (2012). “Review of highway runoff characteristics: Comparative analysis and universal implications.” Water Res., 46(20), 6609–6624.</ref>). |
| + | |||
| + | ===Phosphorus levels in stormwater=== | ||
| + | The total phosphorus concentrations in stormwater runoff depend on the type of land use and range in 0.16-0.46 mg/L (Maestre and Pitt 2005<ref name="example1" />). Stormwater features should reduce these nutrient concentrations before reaching receiving streams and lakes. Environment Canada (2004<ref name="example2">Environment Canada. (2004). Canadian guidance framework for the management of phosphorus in freshwater systems. Ecosystem Health: Science‐based solutions report no. 1–8. Cat. No. En1–34/8–2004E. </ref>) indicates a range of 0.001-2 mg/L (1-200 µg/L) for concentration of total phosphorus in natural waters, while the same range for uncontaminated freshwaters is within 0.01-0.05 mg/L (10-50 µg/L). Within lakes and rivers, trigger concentration ranges are identified and used internationally to explain trophic status of these waters. Based on these triggers, Environment Canada has identified the acceptable range of nutrients as 0.01-0.035 mg/L (10-35 µg/L). Given the flow of water within streams and capacity to flush out pollutants, rivers can maintain higher phosphorus loads than lakes without alterations of community composition and biomass (Environment Canada, 2004<ref name="example2" />). Stormwater features often drain into streams and therefore a similar outflow concentration ranges for total phosphorus is expected from those. | ||
| + | |||
| + | ==Test methods to estimate phosphorus levels in water & soil== | ||
| + | Phosphorus concentrations mentioned above relate to the phosphorus content in water. Depending on the LID type, phosphorus exists in the soil/media as well. Such LIDs include, [[bioretention]], [[enhanced swales|enhanced grass swales]], [[vegetated filter strips]], [[absorbent landscapes]], and [[green roofs]]. The methods used for phosphorus concentration estimation in both water and soil are summarized below. | ||
| + | |||
| + | Measuring the phosphorus concentrations in stormwater is important for performance evaluation/inspection and ensuring that outflow to the streams meets the mentioned concentration requirements. For performance evaluation, both inflow and outflow of a stormwater feature should be sampled. Sampling of just outflow reveals the concentrations entering streams and checks if they meet the requirements. There are several sampling methods, decision on the most appropriate method to use, depends on the sampling objectives and budget limitations. | ||
| + | #'''Grab sampling''' is the least expensive method and often does not yield accurate and representative results. Depending on the timing of the grab sample, concentrations may be too high or too low as they change quickly within a rain event. | ||
| + | #'''Automated samplers''' used to collect water from a rain event at given intervals (time or flow volume) are generally the most popular sampling method and involve compositing rain events to estimate an even mean concentration (EMC). For further information on sampling methods refer to the [https://sustainabletechnologies.ca/events/webinar-real-time-water-quality-monitoring-guide/ STEP Real-Time Water Quality Monitoring – How-To Guide]. The collected samples should be tested by a verified laboratory and handled based on laboratory instruction. | ||
| + | |||
| + | Measuring phosphorus concentration in filter media and growing media, is important for assumption and/or verification inspections. Such media contains phosphorus to support plant growth; however, the amount of phosphorus should remain low to avoid substantial leaching and nutrient contribution to nearby receiving waters. The organic matter in the media, as well as deceased plants can decompose and release both organic and inorganic phosphorus. This can increase concentrations in outflow, rendering LID facilities an exporter of nutrients instead of a treatment feature (Bratieres et al. 2008<ref>Bratieres, K., Fletcher, T. D., Deletic, A., & Zinger, Y. A. R. O. N. (2008). Nutrient and sediment removal by stormwater biofilters: A large-scale design optimization study. Water research, 42(14), 3930-3940</ref>). Therefore, it is important to measure the phosphorus content of LID media and/or bulk materials such as [[compost]], and [[topsoil]] and ensure that it is within appropriate design specification range. '''Extractable phosphorus''' is the portion of soil phosphorus that is easily available to organisms like plant and algae and is of immediate concern to water quality in large amounts, so should be included in the parameters tested. '''Extractable phosphorus''' testing is commercially available from soil testing laboratories servicing agricultural and horticultural industries. The level of phosphorus saturation in a filter or growing media can also be evaluated as Phosphorus Saturation Index (PSI). PSI is the proportion of extractable phosphorus to extractable aluminum and iron in the soil sample. | ||
| + | |||
| + | ==Limiting excess phosphorus== | ||
| + | ===Volume reduction=== | ||
| + | In many forms of LID practice, the dominant mechanism reducing the loading of phosphorus, is the significant volume reduction of the outflow achieved. | ||
| + | |||
| + | ===Chemical control=== | ||
| + | For infiltration practices an 'amendment' or chemically reactive 'additive' can help to retain even more phosphorus. Typically these components would make up 5 to 10% by volume of the filter media mixture. | ||
| + | {{:Additives}} | ||
| + | |||
| + | ==Phosphorus testing in filter media== | ||
| + | To help ensure LID BMPs sustain healthy vegetation cover while not contributing substantially to nutrient loading of receiving waters, the quantity of '''extractable (i.e., available) phosphorus''' in the filter media or growing media (i.e., soil) component needs to be measured and compared to design specifications or acceptance criteria. | ||
| + | {{:Phosphorus testing in media}} | ||
| + | |||
| + | The levels of phosphorus saturation in a filter or growing media can also be evaluated and discussed as Phosphorus Saturation Index (PSI). PSI is the proportion of extractable phosphorus to extractable aluminum and iron in the soil sample. | ||
| + | |||
| + | ==Design and maintenance considerations== | ||
| + | The mechanisms for phosphorus removal are sedimentation, filtration, adsorption, [[Understanding rainfall statistics|precipitation]], and [[Plants#Plant Characteristics|plant uptake]]. Particulate phosphorus can be removed through sedimentation and filtration and is often trapped among other solids within a shallow depth at the media surface (Hsieh et al. 2007<ref name="example3">Hsieh, C.-H., Davis, A. P., and Needelman, B. A. (2007).“Bioretentioncolumn studies of phosphorus removal from urban stormwater runoff.” Water Environ. Res., 79(2), 177–184.</ref>). Dissolved phosphorus is removed deeper in the media as it requires higher retention time (Hsieh et al. 2007<ref name="example3" />). Hunt et al. (2006)<ref name="example4">Hunt, W. F., Jarrett, A. R., Smith, J. T., and Sharkey, L. J. (2006).“Evalu-ating bioretention hydrology and nutrient removal at three field sites in North Carolina.” J. Irrig. Drain. Eng., 132(6), 600–608.</ref>, suggested a minimum depth of 0.6m and recommends 0.9m and infiltration rate of 0.007- 0.028 mm/s (1-4 in/h) for targeted removal of dissolved phosphorus. Removal of dissolved phosphorus relies heavily on the specifications of the LID media, it’s phosphorus content, type and percentage of organic matter, its potential hydrogen (pH), and temperature. | ||
| + | |||
| + | Media with high phosphorus content provides additional support for plant growth, however it will harm the phosphorus removal capability of the feature (Hunt et al. 2006)<ref name="example4" />). Similarly high percentage of organic matter in the media can increase the phosphorus content after degradation and lead to leaching of phosphorus (Clark and Pitt 2009<ref>Clark, S. E., and Pitt, R. (2009).“Storm-water filter media pollutantretention under aerobic versus anaerobic conditions.” J. Environ.Eng., 135(5), 367–371.</ref>). Different types of organic matter have various degrees of phosphorus leaching. To ensure that an LID can provide phosphorus removal, the phosphorus content and percentage of organic matter must be carefully selected and implemented during construction. For proper ranges of these values refer to the [[Bioretention: Filter media|bioretention media page]]. The suggested ranges should be met during the design phase and inspected before assumption of the feature. | ||
| + | |||
| + | A maintenance strategy common to all types of LID practices to avoid nutrient leaching is annual removal of accumulated sediment and debris from inlets. For bioretention cells, bioswales and stormwater tree trenches featuring surface inlets and soil media, periodic removal of the top 2 to 5 centimetres of media in areas adjacent to inlets, and replacement with material that meets design specifications has also been recommended.<ref> Johnson, J.P., Hunt, W.F. 2016. Evaluating the spatial distribution of pollutants and associated maintenance requirements in an 11 year-old bioretention cell in urban Charlotte, NC. Journal of Environmental Management. 184 (2016):363-370. https://www.sciencedirect.com/science/article/pii/S0301479716307812 </ref> <ref>Jones, P.S., Davis, A.P. 2013. Spatial Accumulation and Strength of Affiliation of Heavy Metals in Bioretention Media. Journal of Environmental Engineering. 139(4): 479-487. https://ascelibrary.org/doi/abs/10.1061/%28ASCE%29EE.1943-7870.0000624 </ref> | ||
| + | |||
| + | ==Additives for enhanced phosphorous removal== | ||
| + | Particulate phosphorus is removed to a good extent in LIDs due to the sedimentation and filtration mechanisms offered by these features. To further improve the removal of total phosphorus, the removal of dissolved phosphorus is targeted. As explained in the previous section, adsorption is the main removal mechanism for dissolved phosphorus and aluminum and iron are the main sorptive elements. Therefore, including [[Additives| additives]] in filter media blends can enhance phosphorus retention. Examples of such [[Additives| additives]] are [[Iron filings (ZVI)|iron filings]] or zero valent iron, iron-enriched or [[red sand|“red” sand]], and [[water treatment residuals]]. Other [[Additives| additives]] that enhance filter media sorption capacity are [[biochar]], [[Bold & Gold]], [[Smart Sponge]], and [[sorbtive media| Sorbtive Media]]. See [[Additives]] page for further details and links. | ||
| + | |||
| + | Determining when additive enhanced filter media needs replacing or maintenance represents a new challenge for stormwater asset managers, as there are no suitable visual indicators. Erickson et al. (2018) suggest effluent sampling and laboratory testing to identify when enhanced filter media pollutant retention is waning, or periodic sampling and batch (laboratory) testing of filter media to directly measure its capacity to retain the targeted pollutants.<ref>Erickson, A.J., Taguchi, V.J., Gulliver, J.S. 2018. The Challenge of Maintaining Stormwater Control Measures: A Synthesis of Recent Research and Practitioner Experience. Sustainability. 2018, 10, 3666. https://www.mdpi.com/2071-1050/10/10/3666 </ref> | ||
| + | |||
| + | ==References== | ||
| + | [[Category:Phosphorus]] | ||
| + | [[Category: Water quality]] | ||
Latest revision as of 17:20, 20 March 2023
Phosphorus in stormwater[edit]
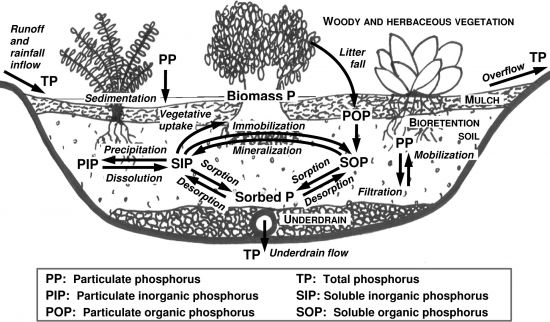
Phosphorus in stormwater exists in both particulate and dissolved forms and is often explained in terms of total phosphorus (TP) and orthophosphate (–PO43-). Total phosphorus is the sum of particulate and dissolved phosphorus and contains both organic and inorganic forms. Particulate phosphorus is often associated with solids and sediments. Dissolved phosphorus consists of mainly inorganic orthophosphate and organic phosphorus. Orthophosphate meanwhile indicates the bioavailable phosphorus.
Phosphorus in stormwater is commonly considered to be primarily available in particulate form. While most studies reported majority of phosphorus (~55%) in particulate form (Maestre and Pitt 2005[1]; Erickson et al, 2012[2]) in some cases the dissolved form may explain up to 90% of the total phosphorus (Erickson et al. 2007[3]; Kayhanian et al. 2012[4]).
Phosphorus levels in stormwater[edit]
The total phosphorus concentrations in stormwater runoff depend on the type of land use and range in 0.16-0.46 mg/L (Maestre and Pitt 2005[1]). Stormwater features should reduce these nutrient concentrations before reaching receiving streams and lakes. Environment Canada (2004[5]) indicates a range of 0.001-2 mg/L (1-200 µg/L) for concentration of total phosphorus in natural waters, while the same range for uncontaminated freshwaters is within 0.01-0.05 mg/L (10-50 µg/L). Within lakes and rivers, trigger concentration ranges are identified and used internationally to explain trophic status of these waters. Based on these triggers, Environment Canada has identified the acceptable range of nutrients as 0.01-0.035 mg/L (10-35 µg/L). Given the flow of water within streams and capacity to flush out pollutants, rivers can maintain higher phosphorus loads than lakes without alterations of community composition and biomass (Environment Canada, 2004[5]). Stormwater features often drain into streams and therefore a similar outflow concentration ranges for total phosphorus is expected from those.
Test methods to estimate phosphorus levels in water & soil[edit]
Phosphorus concentrations mentioned above relate to the phosphorus content in water. Depending on the LID type, phosphorus exists in the soil/media as well. Such LIDs include, bioretention, enhanced grass swales, vegetated filter strips, absorbent landscapes, and green roofs. The methods used for phosphorus concentration estimation in both water and soil are summarized below.
Measuring the phosphorus concentrations in stormwater is important for performance evaluation/inspection and ensuring that outflow to the streams meets the mentioned concentration requirements. For performance evaluation, both inflow and outflow of a stormwater feature should be sampled. Sampling of just outflow reveals the concentrations entering streams and checks if they meet the requirements. There are several sampling methods, decision on the most appropriate method to use, depends on the sampling objectives and budget limitations.
- Grab sampling is the least expensive method and often does not yield accurate and representative results. Depending on the timing of the grab sample, concentrations may be too high or too low as they change quickly within a rain event.
- Automated samplers used to collect water from a rain event at given intervals (time or flow volume) are generally the most popular sampling method and involve compositing rain events to estimate an even mean concentration (EMC). For further information on sampling methods refer to the STEP Real-Time Water Quality Monitoring – How-To Guide. The collected samples should be tested by a verified laboratory and handled based on laboratory instruction.
Measuring phosphorus concentration in filter media and growing media, is important for assumption and/or verification inspections. Such media contains phosphorus to support plant growth; however, the amount of phosphorus should remain low to avoid substantial leaching and nutrient contribution to nearby receiving waters. The organic matter in the media, as well as deceased plants can decompose and release both organic and inorganic phosphorus. This can increase concentrations in outflow, rendering LID facilities an exporter of nutrients instead of a treatment feature (Bratieres et al. 2008[6]). Therefore, it is important to measure the phosphorus content of LID media and/or bulk materials such as compost, and topsoil and ensure that it is within appropriate design specification range. Extractable phosphorus is the portion of soil phosphorus that is easily available to organisms like plant and algae and is of immediate concern to water quality in large amounts, so should be included in the parameters tested. Extractable phosphorus testing is commercially available from soil testing laboratories servicing agricultural and horticultural industries. The level of phosphorus saturation in a filter or growing media can also be evaluated as Phosphorus Saturation Index (PSI). PSI is the proportion of extractable phosphorus to extractable aluminum and iron in the soil sample.
Limiting excess phosphorus[edit]
Volume reduction[edit]
In many forms of LID practice, the dominant mechanism reducing the loading of phosphorus, is the significant volume reduction of the outflow achieved.
Chemical control[edit]
For infiltration practices an 'amendment' or chemically reactive 'additive' can help to retain even more phosphorus. Typically these components would make up 5 to 10% by volume of the filter media mixture. Design innovations to improve water quality treatment performance of filter media mixtures involve the incorporation of additives to enhance retention of reactive or dissolved pollutants. A number of granular amendments have been demonstrated to improve nutrient removal from discharge water in BMPs such as bioretention systems, stormwater planters, absorbent landscapes, sand filters or green roofs.
There are two primary processes involved, chemical precipitation and adsorption. Both mechanisms are ultimately finite, but have been shown in some cases to make significant improvements on the discharged water quality over several years. For instance, a two year STEP research study that compared standard bioretention media, to the same media amended with Sorbtive™ in one plot and iron enriched sand (aka red sand) in another showed statistically significant improvements in effluent phosphorus concentrations from the two media amended plots (STEP, 2019)[7].
Determining when additive enhanced filter media needs replacing or maintenance represents a new challenge for stormwater asset managers, as there are no suitable visual indicators. Erickson et al. (2018) suggest effluent sampling and laboratory testing to identify when enhanced filter media pollutant retention is waning, or periodic sampling and batch (laboratory) testing of filter media to directly measure its capacity to retain the targeted pollutants[8]. Periodic replacement of filter media at inlet locations should be considered as an operation and maintenance best practice to maintain treatment performance.
In our effort to make this guide as functional as possible, we have decided to include proprietary systems and links to manufacturers websites.
Inclusion of such links does not constitute endorsement by the Sustainable Technologies Evaluation Program.
Lists are ordered alphabetically; link updates are welcomed using the form below.
| Material | Benefits | Potential concerns |
|---|---|---|
| Biochar | Renewable Enhances soil aggregation, water holding capacity and organic carbon content |
Currently expensive Energy intensive to produce Some sources say ineffective for phosphorus removal |
| Bold & GoldTM | Documented total phosphorus removal of up to 71%[9] | Proprietary |
| Iron filings or Zero valent iron (ZVI) | Proven phosphorus retention Retained phosphorus is stable |
May harm plants[10] Removal efficiency declines with increased concentration of incoming phosphorus |
| Red sand or Iron-enriched sand | Proven phosphorus removal Also removes TSS |
Poor orthophosphate removal in hypoxic or anoxic conditions |
| Smart SpongeTM | Removes phosphorus, as well as TSS, fecal coliform bacteria and heavy metals Non-leaching |
1-3 year lifespan, after which the product is removed as solid waste Proprietary |
| Sorbtive MediaTM | High phosphorus removal efficiency | Proprietary |
| Water treatment residuals | Waste product reuse | Quality control (capabilities depend on source, treatment methods, storage time) |
Phosphorus testing in filter media[edit]
To help ensure LID BMPs sustain healthy vegetation cover while not contributing substantially to nutrient loading of receiving waters, the quantity of extractable (i.e., available) phosphorus in the filter media or growing media (i.e., soil) component needs to be measured and compared to design specifications or acceptance criteria. For BMPs including: bioretention, enhanced grass swales, vegetated filter strips, absorbent landscapes, green roofs and, bulk materials including: compost, and topsoil, phosphorus (P) should be measured as extractable phosphorus. Extractable phosphorus is the portion that is easily available to organisms like plants and algae, so the parameter of immediate concern to water quality.
The quantity of extractable phosphorus is determined through acid or base extraction of a sample and testing the concentration in solution by a soil testing laboratory. Commonly used extraction methods on soil samples are the Bray and Kurtz P-1 procedure for non-calcareous soil [11]or the Sodium Bicarbonate (Olsen) method for calcareous soil [12].
The Olsen method is recommended as the default to use for typical Ontario soils [13]. Calcareous soils are mostly or partly composed of calcium carbonate (i.e., lime or limestone). The Olsen extraction method should be used if the soil contains more than 2% calcium carbonate [14]
For green roof media, the Saturated Media Extract (SME) method should be used [15]. In this extraction procedure, a sample of the media is saturated with deionized water containing a small amount of Pentetic acid (DTPA) to enhance extraction of micro-nutrients [16]. This SME procedure should also be used to measure concentrations of soluble salts and nitrogen in green roof growing media.
Inspections[edit]
Construction inspections[edit]
If laboratory testing indicates the extractable phosphorus concentration is not within the design or product specification range, notify the supplier, issue a “do not install” order to the construction site supervisor and contact the design professionals and property owner or project manager to determine corrective actions.
Assumption and Verification inspections[edit]
For filter media or growing media found through testing to be below the design or product specification range, or Acceptance Criteria range, corrective actions are only needed if problems with vegetation cover, condition or composition (i.e., dominance by weeds) are also detected through visual inspection. Where vegetation cover is poor, unhealthy or dominated by weeds and soil P is lower than the design specification or Acceptance Criteria, schedule investigative work to do further sampling and testing to determine the affected area and depth and decide on corrective actions. Depending on the findings, corrective action could involve amending the soil with compost or other fertilizer.
Amendments to green roof growing media to address P deficiency should be prescribed by the media manufacturer or product vendor. Where soil P concentration is found to be higher than the Acceptance Criteria range, and the BMP drains to a nutrient sensitive receiving water, continuous monitoring during natural or simulated storm events should be undertaken that includes sampling and testing of nutrient concentrations (i.e., Phosphorus and Nitrogen) in sub-drain or surface flows from the BMP to evaluate if the exceedance is negatively impacting effluent quality and if corrective actions are warranted. Corrective action could involve incorporating a soil additive that increases phosphorus retention, or replacement of part or all of the media or topsoil with material that is within the design or product specification.
The levels of phosphorus saturation in a filter or growing media can also be evaluated and discussed as Phosphorus Saturation Index (PSI). PSI is the proportion of extractable phosphorus to extractable aluminum and iron in the soil sample.
Design and maintenance considerations[edit]
The mechanisms for phosphorus removal are sedimentation, filtration, adsorption, precipitation, and plant uptake. Particulate phosphorus can be removed through sedimentation and filtration and is often trapped among other solids within a shallow depth at the media surface (Hsieh et al. 2007[18]). Dissolved phosphorus is removed deeper in the media as it requires higher retention time (Hsieh et al. 2007[18]). Hunt et al. (2006)[19], suggested a minimum depth of 0.6m and recommends 0.9m and infiltration rate of 0.007- 0.028 mm/s (1-4 in/h) for targeted removal of dissolved phosphorus. Removal of dissolved phosphorus relies heavily on the specifications of the LID media, it’s phosphorus content, type and percentage of organic matter, its potential hydrogen (pH), and temperature.
Media with high phosphorus content provides additional support for plant growth, however it will harm the phosphorus removal capability of the feature (Hunt et al. 2006)[19]). Similarly high percentage of organic matter in the media can increase the phosphorus content after degradation and lead to leaching of phosphorus (Clark and Pitt 2009[20]). Different types of organic matter have various degrees of phosphorus leaching. To ensure that an LID can provide phosphorus removal, the phosphorus content and percentage of organic matter must be carefully selected and implemented during construction. For proper ranges of these values refer to the bioretention media page. The suggested ranges should be met during the design phase and inspected before assumption of the feature.
A maintenance strategy common to all types of LID practices to avoid nutrient leaching is annual removal of accumulated sediment and debris from inlets. For bioretention cells, bioswales and stormwater tree trenches featuring surface inlets and soil media, periodic removal of the top 2 to 5 centimetres of media in areas adjacent to inlets, and replacement with material that meets design specifications has also been recommended.[21] [22]
Additives for enhanced phosphorous removal[edit]
Particulate phosphorus is removed to a good extent in LIDs due to the sedimentation and filtration mechanisms offered by these features. To further improve the removal of total phosphorus, the removal of dissolved phosphorus is targeted. As explained in the previous section, adsorption is the main removal mechanism for dissolved phosphorus and aluminum and iron are the main sorptive elements. Therefore, including additives in filter media blends can enhance phosphorus retention. Examples of such additives are iron filings or zero valent iron, iron-enriched or “red” sand, and water treatment residuals. Other additives that enhance filter media sorption capacity are biochar, Bold & Gold, Smart Sponge, and Sorbtive Media. See Additives page for further details and links.
Determining when additive enhanced filter media needs replacing or maintenance represents a new challenge for stormwater asset managers, as there are no suitable visual indicators. Erickson et al. (2018) suggest effluent sampling and laboratory testing to identify when enhanced filter media pollutant retention is waning, or periodic sampling and batch (laboratory) testing of filter media to directly measure its capacity to retain the targeted pollutants.[23]
References[edit]
- ↑ 1.0 1.1 Maestre, A., and Pitt, R. (2005). “The National Stormwater Quality Database, Version 1.1 a compilation and analysis of NPDES stormwater monitoring information.” U.S. Environmental Protection Agency (EPA) Office of Water, Washington, DC.
- ↑ Erickson, A. J., Gulliver, J. S., and Weiss, P. T. (2012). Capturing dissolved phosphorus with iron enhanced sand filtration. Water Res., 46(9), 3032–3042.
- ↑ Erickson, A., Gulliver, J. S., and Weiss, P. T. (2007). “Enhanced sand filtration for storm water phosphorus removal.” J. Environ. Eng., 10.1061/(ASCE)0733-9372(2007)133:5(485), 485–497.
- ↑ Kayhanian, M., Fruchtman, B., Gulliver, J. S., Montanaro, C., Raniere, E., and Wuertz, S. (2012). “Review of highway runoff characteristics: Comparative analysis and universal implications.” Water Res., 46(20), 6609–6624.
- ↑ 5.0 5.1 Environment Canada. (2004). Canadian guidance framework for the management of phosphorus in freshwater systems. Ecosystem Health: Science‐based solutions report no. 1–8. Cat. No. En1–34/8–2004E.
- ↑ Bratieres, K., Fletcher, T. D., Deletic, A., & Zinger, Y. A. R. O. N. (2008). Nutrient and sediment removal by stormwater biofilters: A large-scale design optimization study. Water research, 42(14), 3930-3940
- ↑ STEP. 2019. Improving nutrient retention in bioretention. Technical Brief. Accessed: https://sustainabletechnologies.ca/app/uploads/2019/06/improving-nutrient-retention-in-bioretention-tech-brief.pdf
- ↑ Erickson, A.J., Taguchi, V.J., Gulliver, J.S. 2018. The Challenge of Maintaining Stormwater Control Measures: A Synthesis of Recent Research and Practitioner Experience. Sustainability. 2018, 10, 3666. https://www.mdpi.com/2071-1050/10/10/3666
- ↑ Hood A, Chopra M, Wanielista M. Assessment of Biosorption Activated Media Under Roadside Swales for the Removal of Phosphorus from Stormwater. Water. 2013;5(1):53-66. doi:10.3390/w5010053.
- ↑ Logsdon SD, Sauer PA. Iron Filings Cement Engineered Soil Mix. Agron J. 2016;108(4):1753. doi:10.2134/agronj2015.0427.
- ↑ Bray, R.H. and Kurtz, L.T. 1945. Determination of total, organic, and available forms of phosphorus in soils. Soil Science, 59: 39-45.
- ↑ Olsen, S.R., Cole, C.V., Watanabe, F.S., and Dean, L.A. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939:1-19. Gov. Printing Office Washington D.C.
- ↑ Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA). 2006. Soil Fertility Handbook. Publication #611. Toronto, Ontario.
- ↑ Frank, K., Beegle, D., Denning, J. 2012. “Phosphorus” In Recommended Chemical Soil Test Procedures for the North Central Region. North Central Regional Research Publication No. 221. Missouri Agricultural Experimental Station.
- ↑ Green Roofs for Healthy Cities. 2011. Advanced Green Roof Maintenance: Participant’s Manual. Toronto, ON.
- ↑ Warnacke, D. 1995. “Recommended Test Procedures for Greenhouse Growth Media.” In J. Thomas Sims and A. Wolf, eds., Recommended Soil Testing Procedures for the Northeastern United States. Northeast Regional Bulletin #493. Newark: Agricultural Experiment Station, University of Delaware.
- ↑ Roy-Poirier, A., Champagne, P., and Filion, Y. 2010. Bioretention processes for phosphorus pollution control. Environmental Reviews, 18(NA), pp.159-173.
- ↑ 18.0 18.1 Hsieh, C.-H., Davis, A. P., and Needelman, B. A. (2007).“Bioretentioncolumn studies of phosphorus removal from urban stormwater runoff.” Water Environ. Res., 79(2), 177–184.
- ↑ 19.0 19.1 Hunt, W. F., Jarrett, A. R., Smith, J. T., and Sharkey, L. J. (2006).“Evalu-ating bioretention hydrology and nutrient removal at three field sites in North Carolina.” J. Irrig. Drain. Eng., 132(6), 600–608.
- ↑ Clark, S. E., and Pitt, R. (2009).“Storm-water filter media pollutantretention under aerobic versus anaerobic conditions.” J. Environ.Eng., 135(5), 367–371.
- ↑ Johnson, J.P., Hunt, W.F. 2016. Evaluating the spatial distribution of pollutants and associated maintenance requirements in an 11 year-old bioretention cell in urban Charlotte, NC. Journal of Environmental Management. 184 (2016):363-370. https://www.sciencedirect.com/science/article/pii/S0301479716307812
- ↑ Jones, P.S., Davis, A.P. 2013. Spatial Accumulation and Strength of Affiliation of Heavy Metals in Bioretention Media. Journal of Environmental Engineering. 139(4): 479-487. https://ascelibrary.org/doi/abs/10.1061/%28ASCE%29EE.1943-7870.0000624
- ↑ Erickson, A.J., Taguchi, V.J., Gulliver, J.S. 2018. The Challenge of Maintaining Stormwater Control Measures: A Synthesis of Recent Research and Practitioner Experience. Sustainability. 2018, 10, 3666. https://www.mdpi.com/2071-1050/10/10/3666
