Difference between revisions of "Winter Management"
Jenny Hill (talk | contribs) m (→De-icing Salt) |
Jenny Hill (talk | contribs) m (→De-icing Salt) |
||
| Line 9: | Line 9: | ||
{{:De-icing}} | {{:De-icing}} | ||
| − | <pdf>LSRCA_snow_pile.pdf</pdf> | + | <pdf>File:LSRCA_snow_pile.pdf</pdf> |
Sodium and chloride ions in de-icing salts applied to asphalt areas travel easily with the runoff water. De-icing salt can increase the mobility of some [[heavy metals]] in soil (e.g. lead, copper or cadmium). This may increase the downstream concentration of these metals <ref>Amrhein, C., Strong, J.E., and Mosher, P.A. 1992. Effect of de-icing salts on metal and organic matter mobilization in roadside soils. Environmental Science and Technology. Vol. 26, No. 4, pp. 703-709</ref><ref>Bauske, B., Goetz, D. 1993. Effects of de-icing salts on heavy metal mobility. Acta Hydrochimica Hydrobiologica. Vol. 21. pp. 38-42., 1993).</ref> | Sodium and chloride ions in de-icing salts applied to asphalt areas travel easily with the runoff water. De-icing salt can increase the mobility of some [[heavy metals]] in soil (e.g. lead, copper or cadmium). This may increase the downstream concentration of these metals <ref>Amrhein, C., Strong, J.E., and Mosher, P.A. 1992. Effect of de-icing salts on metal and organic matter mobilization in roadside soils. Environmental Science and Technology. Vol. 26, No. 4, pp. 703-709</ref><ref>Bauske, B., Goetz, D. 1993. Effects of de-icing salts on heavy metal mobility. Acta Hydrochimica Hydrobiologica. Vol. 21. pp. 38-42., 1993).</ref> | ||
Revision as of 13:50, 10 November 2017
Cold Climate[edit]
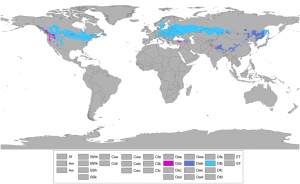
The majority of Ontario has a "Humid continental" Dfb climate, which includes average temperatures in the coldest month well below -3°C. This condition is found in many other parts of the world where LID strategies are routinely employed, including many northern states in the USA and a large swath of northern Europe. Common concerns associated with the use of LID during out
De-icing Salt[edit]
The URL or file path LSRCA_snow_pile.pdf does not exist.
Sodium and chloride ions in de-icing salts applied to asphalt areas travel easily with the runoff water. De-icing salt can increase the mobility of some heavy metals in soil (e.g. lead, copper or cadmium). This may increase the downstream concentration of these metals [1][2]
http://www.unh.edu/unhsc/sites/unh.edu.unhsc/files/pubs_specs_info/jee_3_09_unhsc_cold_climate.pdf
Very few studies have sampled groundwater below infiltration facilities or roadside ditches receiving de-icing salt laden runoff have found concentrations of heavy metals that exceed drinking water standards [3][4]
To minimize risk of groundwater or soil contamination, the following management approaches are recommended (Pitt et al., 1999; TRCA, 2009b):
Stormwater infiltration practices should not receive runoff from the following areas:
- Where large amounts of de-icing salts are applied (e.g., busy highways), or
- Pollution hot spots (e.g. vehicle fuelling, servicing or demolition areas, outdoor storage or handling areas for hazardous materials, and some heavy industry sites); *Prioritize infiltration of runoff from source areas that are comparatively less contaminated such as roofs, low traffic roads and parking areas; and
- Apply pretreatment practices before infiltration of road or parking area runoff.
[edit]
Rainwater harvesting
Freezing temperatures can cause problems with pipes and cisterns exposed above the frost penetration line. This maybe a significant issue for rainwater harvesting systems, including residential rain barrels.
Green Roofs
The survival of green roof planting is greater in winters with long deep sub-zero temperatures. Being shallow and very exposed to warming sunlight, green roofs thaw rapidly. Frequent freeze-thaw cycles in the early and late winter are associated higher loss of vegetation on green roofs.
- ↑ Amrhein, C., Strong, J.E., and Mosher, P.A. 1992. Effect of de-icing salts on metal and organic matter mobilization in roadside soils. Environmental Science and Technology. Vol. 26, No. 4, pp. 703-709
- ↑ Bauske, B., Goetz, D. 1993. Effects of de-icing salts on heavy metal mobility. Acta Hydrochimica Hydrobiologica. Vol. 21. pp. 38-42., 1993).
- ↑ Howard, K.W.F. and Beck, P.J. 1993. Hydrogeochemical implications of groundwater contamination by road de-icing chemicals. Journal of Contaminant Hydrology. Vol. 12. pp. 245-268.
- ↑ Granato, G.E., Church, P.E., Stone, V.J. 1995. Mobilization of Major and Trace Constituents of Highway Runoff in Groundwater Potentially Caused by De-icing Chemical Migration. Transportation Research Record. No. 1483.