Nutrients
Overview[edit]
Nutrients are an essential part of life, however excess amounts of them can harm the environment. Anthropogenic activities can lead to such excessive amount of nutrients in runoff and cause degradation of receiving waters. Phosphorus and Nitrogen are the main nutrients of concern in stormwater runoff. This page provides information on the sources of nutrients, the issues caused by their excess amounts, and strategies to manage them.
Nutrient pollution[edit]
The primary concern with excess amounts of nutrients is eutrophication (U.S. EPA 1999[2]). Eutrophication is the process of excessive algae growth due to abundance of nutrients that can cause hypoxic condition in water. Excessive algal growth can cover the surface of water and prevent light penetration. Without light, the desirable aquatic plants die off. The eventual decay of aquatic plants and algae, consumes the dissolved oxygen in the water and creates hypoxic condition that can kill fish and other aquatic animals.
Nutrient pollution has been noted as a critical environmental, public health, and economic concern in Canada, Great Lakes, Lake Winnipeg, Lac St. Charles, Lake Simcoe and other water bodies (CWN, 2017[3]). Nutrient pollution can cause environmental imbalance, pose a public health risk for drinking water (USEPA, 2009[4]) and swimming, as inhalation of algae by swimmers or pet can cause hypoxic condition. It can also affect the economy by harming fisheries, tourism, recreation, and increase the treatment cost of drinking water. Historical algal bloom in the Lake Erie during 1960s caused a substantial decline of native aquatic species (CWN, 2017[3]). In 2015 more than 5,000 km2 of Lake Erie was covered by algal blooms rendering the water unsuitable for drinking for residents of Pelee Island, Ontario and Toledo, Ohio (CWN, 2017[3]). In 2011 and 2014, severe nutrient pollution in this lake caused service interruption, equal to $71 million and $65 million USD (Bingham, Sinha, & Lupi, 2015[5]). Reports of nutrient pollution are increasing across Canada causing more beach closures and decline in water quality and fisheries (CWN, 2017[3]).
Excessive algal growth requires both phosphorus and nitrogen. While in some environments nitrogen is considered to be the limiting nutrient that controls such growth, phosphorus has been considered as the main limiting factor in most freshwaters (Howarth & Marino, 2006[6]; Schindler et al., 2016[7]). Therefore, controlling the load of phosphorous leaving a sub-watershed can reduce the chances of nutrient pollution in receiving surface waters. Formation of policies such as Lake Simcoe Phosphorous Offsetting Policy (LSPOP) are examples of such an approach. LSPOP requires a Zero Export Target where all new developments should control 100% of phosphorus from leaving the property (LSRCA, 2021[8]).
In addition to contaminating surface waters, abundance of nutrients can also contaminate the ground waters. Contrary to surface waters, nitrogen is the primary nutrient of concern regarding groundwater quality. Hobbie, et al. (2017[9]) reported that only 22 % of phosphorous is retained within its watershed, while the same estimated for nitrogen is 80%. Therefore, most of the nitrogen is leached in the groundwater or transformed through denitrification. Phosphorus retained in the watershed is often in particle form. Therefore, it tends to be bound to soil particles and retained in the vadose zone (area between ground surface and the groundwater table). However, nitrogen is often available in dissolved form which is more mobile and bioavailable. Thus, it can travel through the vadose zone and contaminate the groundwater.
Nutrient management[edit]
Due to their differing stoichiometry, managing nitrogen pollution is different from that of phosphorous. To reduce the nitrogen pollution, the watershed inputs should be reduced in general, while reducing phosphorus pollution would require reducing the movement of phosphorus from the contributing areas in the watershed (Hobbie et al., 2017[9]).
The key method for nutrient management is source control, or prevention of pollutants from entering stormwater, at the source. Low Impact Development (LID) features are source control measures in this way. Source control policies are cost effective tools for nutrient management (Marsalek and Viklander, 2011[10]) that support and encourage the implementation of LIDs. Examples of such policies are adapted by Lake Simcoe Region Conservation Authority, South Nation Conservation (SNC), Nottawasaga Valley Conservation Authority (NVCA), and Halton Region in Ontario, as well as Chesapeake Bay and Mississippi River Basin (Region of Waterloo, 2017[11]), municipalities as nutrient management by-laws, provinces like Ontario Nutrient Management Act and the Great Lakes Protection Act, and countries like Great Lakes Agreement between Canada and the US (CWN, 2017[3]). Additionally, design guidelines such as this one, provide tools for design and implementation of LIDs. While historically these guidelines indicated percent removal rates, the recent approaches are guiding to meet specific numeric objectives such as concentration (Clark and Pitt, 2012[12]).
Selection of the LID type and its implementation for proper nutrient management, requires a good understanding of site limitations, sources of nutrients, their forms (particulate/soluble), and nutrient removal mechanisms associated with each LID type. The sources of nutrients and removal mechanisms are reviewed in this page. See Phosphorus page for additional information on managing phosphorus.
Nutrient sources[edit]
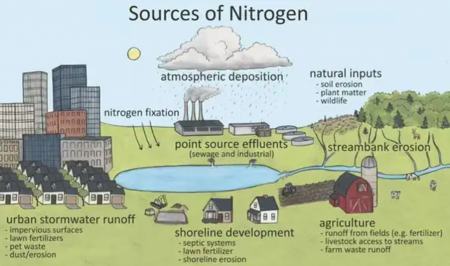
The extreme amounts of nutrients are both due to the general increase of their availability and the decreased removal capacity of natural systems. Following the important technological advancements in early 20th century (Haber-Bosch process), nitrogen in the form of ammonia has increased significantly due to the ongoing production of fertilizers. While the increase in agricultural production has been a beneficial effect of this finding, it has also led to excessive amounts of nutrient source loading. The decrease in nutrient retention capacity is due to the modification of natural systems such as the increased impervious surfaces along with reduction of vegetation, stream channelization and modification and degradation of riparian zones (Collins et al, 2010[14]).
Sources of nutrients are urban surfaces, agricultural activities, as well as the atmosphere itself. Among different urban surfaces, turfs, lawns, and gardens have a high contribution to nutrients in stormwater (Muller et al., 2020[15]). The grass clippings and application of fertilizer and pesticide, renders lawns and turfs as one of the main contributors of total and dissolved phosphorus in stormwater (Muller et al, 2020). Additionally, fallen vegetation foliage is another contributor of a watershed’s nutrient output and specially phosphorus. Selbig, 2016 has reported foliage, contributing to more than 50% of annual phosphorus loads, excluding winter season. The salt used for de-icing in in cold climate areas may contain impurities that carries nitrogen and phosphorus (Muller et al, 2020[15]). Other activities in urban areas such as construction, fuel deposition by vehicles, and leaking of sewer pipes or septic tanks can also contribute to overall nutrient pollution. Similar to urban landscapes, agricultural activity contributes to the nutrient loads by fertilizer and pesticide applications, as well as manure. The nutrient loads from agricultural activities are more significant than urban landscapes. Atmospheric deposition is another source of nutrients, where the atmosphere is rather a carrier than a source of aerosol nutrients created by industrial, or transportation activities (Muller et al, 2020[15]).
Nutrient removal mechanisms[edit]
Nutrients within stormwater can be in particulate or dissolved form. While phosphorus and nitrogen are carried in both forms in stormwater, the majority of phosphorus is particulate bound (approximately 55% [Erickson et al., 2012][16]) and majority of nitrogen is dissolved (Taylor et al. 2005[17]). The stormwater pollution is often attributed to particles. However, the effect of dissolved pollutants on the loads is being realized, as they are more mobile and bioavailable, and therefore can have a quicker effect on the receiving waters (LeFevre et al, 2015[18]).
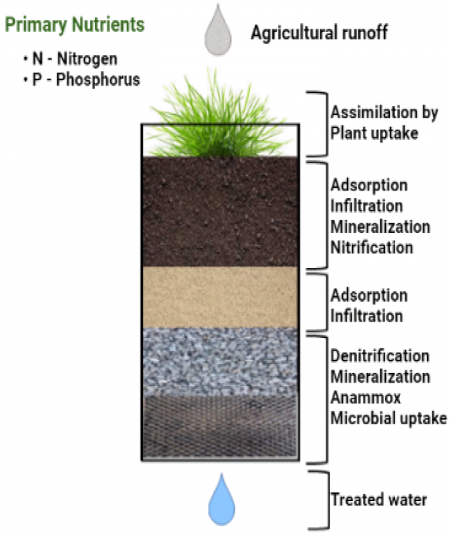
The removal mechanisms depend on the nutrient’s form and can be explained in three categories of physical, chemical, and biological processes. Physical mechanisms are sedimentation and filtration. Chemical mechanisms include adsorption and precipitation. The chemical processes often transform or attach dissolved forms into insoluble solids, which can then be removed by physical processes. Biological processes are those vital for livelihood of organisms such as plant uptake and nitrification/denitrification. A detailed description of each removal mechanism is provided below:
- Sedimentation – As water slows down within a stormwater feature, the suspended particles settle at the bottom, leaving a rather clear water on top. Sedimentation often removes larger particles and particle-bound pollutants.
- Filtration – As water passes through vegetation or filter media of an LID, some pollutants are caught within their structure. Filtration is the mechanism that removes finer particles and pollutants bound to those.
- Adsorption – Through adsorption, the pollutants in the dissolved form are attached to the surfaces of solids due to electrical attraction. This results in the creation of a molecular layer on the surface of the adsorbent solid. The constituents of the solid may also dissolve and return to soluble form.
- Precipitation – Precipitation refers to the chemical reactions that convert dissolved substances into solids. Such solids can then be removed from stormwater by physical processes of sedimentation and filtration.
- Plant uptake – Plant uptake or assimilation is the removal of nutrients from stormwater by the plant roots for growth purposes. While during the growth season, plants consume nutrients and remove them from stormwater, this mechanism is inactive during their dormant seasons. Additionally, when plants die off or their clippings are left in the feature, the nutrients can return to the system. Therefore, it is important to consider removal of the plant litter from the feature, to avoid any additional contribution to the outflow pollution.
- Nitrification/denitrification – This is a microbial process where ammonia is converted to nitrite and then to nitrate by nitrifying bacteria. Through the denitrification process, the nitrate is further converted into gaseous nitrogen. This process is carried by denitrifying bacteria and requires anaerobic conditions. Anaerobic condition, can occur in lower depths of an LID, given the saturated conditions last long enough to minimize oxygen concentrations. Both processes require presence of organic matter as a source of energy.
Each LID practice has the potential to offer one or all the mentioned removal mechanisms. In practice, the chemical and biological removal mechanisms each require favorable environments for activation. These environmental factors include oxygen availability, percentage of available organic matter, potential hydrogen (pH), salinity, and temperature. Please refer to the phosphorus and nitrogen pages for further details.
Additionally, adequate maintenance of LID practices is needed to maintain the nutrient removal capacity of the facility and ensure that it does not become an exporter of nutrients itself. A strategy common to all types of LID practices to avoid nutrient leaching is annual removal of accumulated sediment and debris from inlets. For bioretention cells, bioswales and stormwater tree trenches featuring surface inlets and soil media, periodic removal of the top 2 to 5 centimetres of media in areas adjacent to inlets, and replacement with material that meets design specifications has also been recommended.[19] [20]
References[edit]
- ↑ Osman, M., Takaijudin, H., Massoudieh, A. and H.W., Goh. 2022. Effects of Vegetation and Saturated Zone in Cascaded Bioretention on Enhancing Nutrient Removal. Environmental Engineering Research, 28(3), p.220154.
- ↑ U.S. Environmental Protection Agency (EPA). (1999). Preliminary data summary of urban storm water best management practices. Pub. No. EPA-821-R-99-012, Washington, DC.
- ↑ 3.0 3.1 3.2 3.3 3.4 CWN (Canadian Water Network). (2017). Nutrient Management—Research Insights for Decision Makers. CWN Report, November, 26.
- ↑ United States Environmental Protection Agency (USEPA), 2009. Managing Stormwater Runoff to Prevent Contamination of Drinking Water. Source Water Protection Practices Bulletin. United States Environmental Protection Agency, Office of Water, Washington, D.C.
- ↑ Bingham, M., Sinha, S. K., & Lupi, F. (2015). Economic benefits of reducing harmful algal blooms in Lake Erie. Retrieved from International Joint Commission website: http://ijc.org/fi les/tinymce/uploaded/Publications/Economic-Benefits-Due-to-Reduction-in-HABs-October-2015.pdf
- ↑ Howarth, R. W., & Marino, R. (2006). Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnology and Oceanography, 51, 364-376. doi:10.4319/lo.2006.51.1_part_2.0364
- ↑ Schindler, D. W., Carpenter, S. R., Chapra, S. C., Hecky, R. E., & Orihel, D. M. (2016). Reducing phosphorus to curb lake eutrophication is a success. Environmental Science & Technology, 50, 8923-8929. doi: 10.1021/acs.est.6b02204.
- ↑ LSRCA (Lake Simcoe Region Conservation Authority). (2021). Phosphorus Offsetting Policy. July). Available at: https://www.lsrca.on.ca/Shared%20Documents/Phosphorus_Offsetting_Policy.pdf.
- ↑ 9.0 9.1 Hobbie, S.E., Finlay, J.C., Janke, B.D., Nidzgorski, D.A., Millet, D.B., Baker, L.A., 2017. Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban water pollution. Proc. Natl. Acad. Sci. U. S. A. 114 (16), 4177–4182.
- ↑ Marsalek, J., Viklander, M., 2011. Controlling contaminants in urban stormwater: Linking environmental science and policy. In: Lundqvist, J. (Ed.), On the Water Front: Selections from the 2010 World Water Week in Stockholm. vol. 101. Stockholm International Water Institute (SIWI), Stockholm.
- ↑ Region of Waterloo. (2017). Phosphorus Offsetting: Review of Existing Ontario Programs and Opportunities. Available at: https://www.regionofwaterloo.ca/en/living-here/resources/Documents/water/projects/wastewater/plan/WS2018V5-Tech_Memo_9A_WWTMP-Phosphorus_Offsetting_2017.PDF
- ↑ Clark, S. E., and Pitt, R. (2012). Targeting treatment technologies to address specific stormwater pollutants and numeric discharge limits. Water Res., 46(20), 6715–6730.
- ↑ YSI Inc./Xylem Inc. 2022. How to Get Your Lab Ready for Harmful Algal Blooms. Authored by: Laboratory Team. Aug 18, 2022. Accessed 26 September 2022.https://www.ysi.com/ysi-blog/water-blogged-blog/2022/08/how-to-get-your-lab-ready-for-harmful-algal-blooms
- ↑ Collins, K.A., Lawrence, T.J., Stander, E.K., Jontos, R.J., Kaushal, S.S., Newcomer, T.A., Grimm, N.B. and Ekberg, M.L.C., 2010. Opportunities and challenges for managing nitrogen in urban stormwater: A review and synthesis. Ecological Engineering, 36(11), pp.1507-1519.
- ↑ 15.0 15.1 15.2 Müller, A., Österlund, H., Marsalek, J., & Viklander, M. (2020). The pollution conveyed by urban runoff: A review of sources. Science of the Total Environment, 709, 136125.
- ↑ Erickson, A. J., Gulliver, J. S., and Weiss, P. T. (2012). Capturing dissolved phosphorus with iron enhanced sand filtration. Water Res., 46(9), 3032–3042.
- ↑ Taylor, G. D., Fletcher, T. D., Wong, T. H. F., Breen, P. F., and Duncan, H. P. (2005). Nitrogen composition in urban runoff—Implications for stormwater management. Water Res., 39(10), 1982–1989.
- ↑ LeFevre, G. H., Paus, K. H., Natarajan, P., Gulliver, J. S., Novak, P. J., & Hozalski, R. M. (2015). Review of dissolved pollutants in urban storm water and their removal and fate in bioretention cells. Journal of Environmental Engineering, 141(1), 04014050.
- ↑ Johnson, J.P., Hunt, W.F. 2016. Evaluating the spatial distribution of pollutants and associated maintenance requirements in an 11 year-old bioretention cell in urban Charlotte, NC. Journal of Environmental Management. 184 (2016):363-370. https://www.sciencedirect.com/science/article/pii/S0301479716307812
- ↑ Jones, P.S., Davis, A.P. 2013. Spatial Accumulation and Strength of Affiliation of Heavy Metals in Bioretention Media. Journal of Environmental Engineering. 139(4): 479-487. https://ascelibrary.org/doi/abs/10.1061/%28ASCE%29EE.1943-7870.0000624